11 | Add to Reading ListSource URL: optn.transplant.hrsa.govLanguage: English - Date: 2014-02-21 16:34:01
|
|---|
12![32. Obligations and reporting requirements of centres This guidance note contains: Refer to principle 10, 11 and 13 Mandatory requirements • Extracts from the HFE Act[removed]as amended) 32. Obligations and reporting requirements of centres This guidance note contains: Refer to principle 10, 11 and 13 Mandatory requirements • Extracts from the HFE Act[removed]as amended)](https://www.pdfsearch.io/img/38f4c42d02db92631cbe92f2e80d0fe6.jpg) | Add to Reading ListSource URL: www.hfea.gov.ukLanguage: English - Date: 2014-10-02 10:22:23
|
|---|
13 | Add to Reading ListSource URL: depts.washington.eduLanguage: English - Date: 2011-11-22 16:54:45
|
|---|
14![11. Donor recruitment, assessment and screening This guidance note contains: Mandatory requirements • Extracts from the HFE Act[removed]as amended) • Extracts from licence conditions • Reference to relevant HFEA D 11. Donor recruitment, assessment and screening This guidance note contains: Mandatory requirements • Extracts from the HFE Act[removed]as amended) • Extracts from licence conditions • Reference to relevant HFEA D](https://www.pdfsearch.io/img/acf864ce53434a33bc2a05c794189f19.jpg) | Add to Reading ListSource URL: www.hfea.gov.ukLanguage: English - Date: 2014-10-02 10:20:38
|
|---|
15 | Add to Reading ListSource URL: www.hfea.gov.ukLanguage: English - Date: 2014-10-02 10:22:52
|
|---|
16![20. Donor-assisted conception This guidance note contains: Refer to principles 2, 4, 5 and 6 Mandatory requirements • Extracts from the HFE Act[removed]as amended) 20. Donor-assisted conception This guidance note contains: Refer to principles 2, 4, 5 and 6 Mandatory requirements • Extracts from the HFE Act[removed]as amended)](https://www.pdfsearch.io/img/ae2d56d0a5ba37e49ef4743b71536d32.jpg) | Add to Reading ListSource URL: www.hfea.gov.ukLanguage: English - Date: 2014-10-02 10:24:44
|
|---|
17![3. Counselling This guidance note contains: Refer to principles 2 and 9 Mandatory requirements • Extracts from the HFE Act[removed]as amended) 3. Counselling This guidance note contains: Refer to principles 2 and 9 Mandatory requirements • Extracts from the HFE Act[removed]as amended)](https://www.pdfsearch.io/img/833d652375ca65fc73913e327347b730.jpg) | Add to Reading ListSource URL: www.hfea.gov.ukLanguage: English - Date: 2014-10-02 10:21:53
|
|---|
18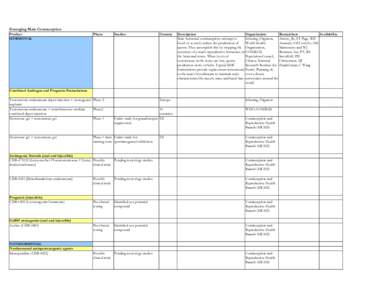 | Add to Reading ListSource URL: www.rhtp.orgLanguage: English - Date: 2008-12-10 17:04:25
|
|---|
19 | Add to Reading ListSource URL: icmr.nic.inLanguage: English - Date: 2011-05-11 03:16:56
|
|---|
20 | Add to Reading ListSource URL: hc-sc.gc.caLanguage: English - Date: 2013-07-12 14:25:40
|
|---|